Abstract
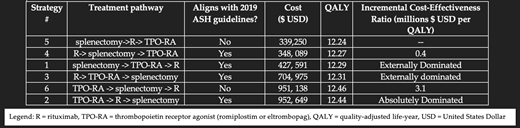
Introduction: Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by accelerated platelet clearance and impaired platelet production. Up to 75% of cases in adults assume a chronic course. Standard second-line treatment options include rituximab, thrombopoietin receptor agonists (TPO-RAs), and splenectomy. The 2019 American Society of Hematology (ASH) guidelines provide three dichomotous evaluations of these treatments with conditional recommendations for 1) splenectomy or TPO-RA, 2) rituximab over splenectomy, and 3) TPO-RA over rituximab. The guideline panel noted that there were no studies available to evaluate the cost-effectiveness of these therapies. We sought to address this knowledge gap by conducting the first cost-effectiveness analysis of second-line therapies for chronic ITP. Methods: We built a Markov model comparing the cost-effectiveness of all six treatment pathways utilizing rituximab, TPO-RA (romiplostim or eltrombopag), and splenectomy. We assumed a median age of 50 at diagnosis and a 20-year time-horizon. Costs were assessed from the health system perspective. Effectiveness was calculated in quality-adjusted life-years (QALYs). The costs of splenectomy treatment included the cost of surgery, postoperative care, accessory spleen imaging and repeat splenectomy, treatment for post-splenectomy sepsis and thromboembolism. The annual risks of post-splenectomy sepsis and thromboembolism were assumed to be the highest reported, every post-splenectomy infectious complication was assumed to be severe septic shock, and patients with thromboembolism accrued the costs of indefinite anticoagulation. To minimize bias against TPO-RAs, TPO-RA therapy was assumed to have no adverse events, a constant high overall response without any loss of effectiveness, and the highest reported rate of successful TPO-RA therapy discontinuation after two years, which we assumed to be permanent. Rituximab treatment was assumed to have no adverse events and overall response rates as previously reported. Cost-effectiveness of each treatment pathway was calculated as the incremental cost-effectiveness ratio (ICER), calculated as ratio of costs to QALYs. The ICER was compared against a 2019 US willingness-to-pay (WTP) threshold of $195,300. We then performed one-way deterministic sensitivity analyses varying all parameters including the costs of splenectomy, TPO-RA, rituximab, splenectomy complications, splenectomy complete response rates, TPO-RA and rituximab overall response rates, utilities of the well and diseases states, annual post-splenectomy septic shock mortality and perioperative splenectomy mortality. We concluded with a probabilistic sensitivity analysis running 10,000 Monte Carlo simulations. Results: The most cost-effective treatment pathway was #5 (splenectomy->rituximab->TPO-RA; Figure). The next most cost-effective pathway was #4 (rituximab followed by splenectomy and then TPO-RA therapy), with an ICER of $369,289. All four remaining treatment pathways (#1-3, 6) utilizing TPO-RA therapy early (first or second) had an ICER above $1 million, far above the US WTP of $195,300, and/or were dominated. Of these, pathways #1 and #3 were externally dominated and #2 was absolutely dominated. No parameter change in one-way deterministic sensitivity analysis in any of the 4 pathways featuring TPO-RA early brought down the ICER to under $1 million. In the probabilistic sensitivity analysis, pathway #5 was favored in 100% of 10,000 Monte Carlo simulations. The cost of TPO-RA would have to be decreased to under $20,000 annually (e.g., >80% reduction in the cost of eltrombopag or romiplostim) before it could become cost-effective in any TPO-early treatment pathway.
Conclusion: Four treatment pathways (#1-#4) are consistent and two pathways (#5-#6) are at variance with the ASH guidelines. Although it does not align with the ASH guidelines, pathway #5 (splenectomy->rituximab->TPO-RA) was most cost-effective. Over a 20-year time-horizon, all pathways featuring early use of a TPO-RA exceeded an ICER >$1 million or were dominated. Because our model was designed to maximize the cost-effectiveness of TPO-RA, it is likely that the actual ICER of pathways featuring early use of TPO-RA are higher than what we report here. Preferred second-line treatment strategies in adults with chronic ITP may be worth considering in light of our findings.
Cuker: Synergy: Consultancy; Novo Nordisk: Research Funding; Alexion: Research Funding; UpToDate: Patents & Royalties; Novartis: Research Funding; Bayer: Research Funding; Takeda: Research Funding; Pfizer: Research Funding; Sanofi: Research Funding; Spark Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal